lother meyers classification
- the alkali metals are going to be at the crest of the curve
- the alkaline earth metal they will occupy the midpoints in descending portion
- transition metals are going to occupy the trough
- the halogens are going to occupy the ascending portion before inert gases
atomic volume =atomic mass/density
long form of periodic table or mosleys pt
- he studies the frequencies of xrays produced by bombardment of beam of electrons of the metal surface
- the square root of frequency is directly proportional to the effective nuclear charge i.e. atomic number


nomenclature of elements above 100
for ex :
sulphur: atomic number = 16
electronic configuration = 1s2 2s2 2p6 3s2 3p6
period = 3
block = p
atomic number = 10+ (no of valence shell electrons)6 = 16
- screening effect of sheilding effect
define : the decrease in force of attraction bw the nucleus and outer electrons by the inner electrons
zeff = z-s (where s is the screening const)
slater's rule
- case 1: if electron in question resides in s or p subshell
rules for case 1
- then all electrons present in shells higher than electron in question contribute zero to sigma or s.
- all electrons present in same shell contribute 0.35 to sigma
- all electrons present in (n-1) shell contribute 0.85 to sigma
- all electrons present in deeper shells contribute 1 to sigma
Q] find the zeff of last electrons of cl
ans] - 1s2 and 2s2 are one orbital all together
- the deeper or innermost orbital is (n-2) for whos electrons contribution will be multiplied to 1
- rest will (n-1)electrons * 0.85 and n electrons * 0.35

find the z eff for chlorine
case 2: if electrons in question is present in d or f subshell
rules for case 2:
- then all electrons present in shells higher than electron in question contribute zero to sigma or s.
- all electrons present in same shell contribute 0.35 to sigma
- all electrons present in (n-1) or deeper shells contribute 1 to sigma
for ex: calculate the effective nuclear charge for iron
ATOMIC RADIUS
define: it is one half of the distance bw two nuclie of like atoms bonded by single covalent bond.
- used for non metals
- CASE 1: for homoatomic molecule the distance bw the two nuclie divided by two
- CASE 2: for heteroatomic molecules and electronegativity almost zero(sum of radium of the two atoms)
- for heteroatomic molecules and the electronegativity is not zero
- if the electronegativity is nearly same we use the stevenson and schoomakerformula
da-b = ra + rb - 0.09|electronegativity difference of both atoms |
- if the electronegativity difference is not zero then we use the porterfeild formula
da-b = ra + rb - 0.09|electronegativity difference of both atoms |2
METALLIC RADIUS
Define: it is one half of the distance bw two nearest neighbouring atom ion metal crystal.
#used for metals
- After studying the three types of radius we can conclude that
Vander waal radius > metallic radius > covalent radius
Q} Hardik pandya went to meet his brother where he saw him studying a chemistry book which lacked the theoretical knowledge of bond lenght of h-f but had the individual radius of u and f as 0.38 and 0.72 A and electronegativity of them as 4 and 2.1 respectively. Find single covalent bond distance from both the formula?
Ans) da-b = ra + rb -0.09|∆x|
Where ra = 0.38
rb = 0.72
∆x = 1.9
The covalent bond radius is 0.93
Similiar for porter field
da-b = ra + rb -0.07 |∆x|2

Numericals
trends in atomic radius
- in grp (top to bottom) = orbit number increase and thus size increases
- in a period (left to right) = effective nuclear charge increases because proton no increases
- atomic radius decreases
IONISATION ENERGY
- The amount of energy required to remove an electron from an isolated gaseous atom.
- The ionisation energy increases as you keep removing electrons.
- If the effective nuclear charge (Z_eff) increases, the attraction increases, thus ionisation energy (IE) increases [Left to Right].
- As the shell number increases, the attraction decreases, and thus IE decreases [Top to Bottom].
- ΔiH is also ionisation energy.
EXCEPTIONS
Inert gases: Inert gases have the largest IE due to their fully filled configuration.
- 2nd and 3rd period elements: Li < B < Be < C < O < N < F [B should have been larger, but it doesn't happen due to half-filled or fully filled configurations].
- Ga and Al: The IE of gallium is more than aluminium due to poor screening of 3d10 electrons, which increases Z_eff, thus increasing attraction and IE.
- 5d > 3d > 4d [due to poor screening of 4f electrons, leading to less repulsion and more attraction].
ELECTRON GAIN ENTHALPY or ELECTRON AFFINITY [deltaegH]
- the amount of energy required or release by gain of an electron from isolated gaseous atom
- if delta h is negative then the rxn is exothermic and energy is released and this electron affinity is more of atom
- if delta h is positive then the rxn is endothermic and this energy absorbed and electron affinity is lesser
- first electron gain enthalpy is negative and second gain enthalpy is positive and after this all the electron gain enthalpy will be positive
- l to right : zeff increases this attraction increases thus electron affinity increases
- top to bottom : size increases thus attraction decreases and this electron affinity increases.
exception:
- 2nd period: be<ne<n<b<li<c<o<f [bete ne na bol kar beti ko fasaya]
[be and ne and n have nearly zero electron affinity]
- 3rd period : mg<ar<al<na<p<si<s<cl [p has vacant 3d which causes higher electron affinity ]
- 3p > 2p due to availibility of vacant 3d orbitals
- halogens: cl>f>br>i [higher cl due to vacant d orbitals]
- oxygen family: s>se>te>po>o [due to compact nature]
- noble gases : electron affinity is always positive because stability is very high.
ELECTRONEGATIVITY(qualitative)
- DEFINATION :the tendency of an atom to attract shared pair of electrons towards itself.
- some scales:
- pauling scale: delta = |χA - χB| = 0.208√Δ,
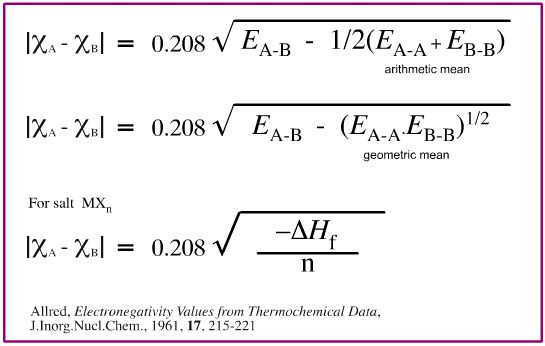
- where E(a-a) is bond energy in k cal/mol.
- mulliken scale: the EN is the average of IE + EA in ex/atom

- relation bw both he scales: ENM / ENP = 2.8
- Allred Ro chow scale formula:
where r is covalent radius and z is effective nuclear charge.
- TRENDS :
- left to right : Zeff is increases thus ENC increases thus EN increases
- top to bottom: Size increases thus outermost sheilding poor thus EN decreases
- hydrogen and phosphorous have the same EN (2.1)
- N and cl also have same EN (3)
- c,i and s also (2.5)
- more positive charge increases EN and more negative charge decreases EN.
underoot (a*b) = a+b/2 [the value]
Application of electronegativity
- to predict the nature of the atom
if one atom has equal electronegativity: then its a covalent bond
if not same : then its a partially ionic and partially covalent bond
HENNY smith formula : 
2.to determine the nature of the oxide
E-O (EXCESS OF H2O) ------- E-O-H
where the bond bw E-O is delta 1 and bond bw O-H is delta
the value Δ = 3.5 - 2.1 = 1.4
Δ1 > 1.4 = bASIC oxide [EOH + H2O = E+OH2 + OH]
Δ1 < 1.4 = acidic oxide [H3O+ + EO-]
TYPES OF OXIDES
- acidic oxides
- solution in H2o is acidic
- react with base
- generally non metallic oxides are acidic
- eg: SO2 + H2O = H2SO3
- basic oxides
- solution in water is basic
- react with acid
- generally metallic oxides
- eg = na2o + h2o = Naoh
- Amphoteric oxides
- both acidic and basic in solution
- react with acid and base depending on the element
- generally metalloids oxide are amphoteric
- eg = AL2O3 + HCL = ALCL3
- al2o3 + naoh = naalo2
- zno + hcl = zncl2
- neutral : doesn't react with acid and base {NO , N2O , CO}
trends of non-metallic character and acidic strenght
- left to right : non-metallic character increases thus acidic strength also increases
- eg: na2o < mgo < al2o3 < p2o5 < so3
- top to bottom : the non-metallic character decreases thus acidic character also decreases
- eg: Na2o3 > k2o > rb2o > cs2o
# for oxides of some element
- acidic strength∝{ no of oxygen / no of central atoms}
- acidic character ∝ oxidation number
- eg : Mno {1/1} , Mn2o3 {3/2}
ACID AND ANHYDRIDES
- keep removing H until oxide is obtained is most cases
- eg: h2co3 = co2
- eg: hno3 = n2o5 {take two moles of hno3}
- eg: h3bo2 = b2o3
ACIDIC STRENGTH IN OXYACIDS
- left to right : non-metallic character increases thus acidic character increases [h3co3<h3po3<h3so4<hclo4]
- top to bottom : non-metallic character decreases this acidic character decreases
# for oxyacids for same central atoms =
A.S ∝ no of oxygen atoms
eg: h2so4> h2so3
exception : [h3po2 > h3po3 > h3po4]
ACIDIC STRENGTH IN HYDRIDE
- left to right : acidic character increases
- top to bottom : acidic character increases
why?
- As you move down a group, the electronegativity of the central atom (e.g., N, P, As, Sb, Bi) decreases. This means the central atom holds the electrons in the bond less strongly, making the bond more polar.
- The bond length between the central atom and hydrogen increases as you go down the group. This weaker bond is easier to break, leading to a greater tendency to release a proton (H+) and thus increased acidity.
- As you move across a period from left to right, the electronegativity of the central atom increases. This makes the E-H bond more polar, with the hydrogen gaining a partial positive charge.
- The more polar the bond, the easier it is for the hydrogen to dissociate as a proton, leading to increased acidity.
basic strength in hydroxide and hydrides
- in hydroxides
- left to right the basic character decreases and metallic character decreases
- top to bottom the mc increases and this the basic strength increases
- eg: lioh > be (oh)2
- lioh < naoh < koh < rboh < rboh ,csoh
- arrhenius base as they release oh
- in hydrides
- left to right the bs decreases so the en decreases
- top to bottom is bs decreases due to increases in size of atom (smaller the size condenser is the electron density thus easier to give out lone pair)











